Abstract
Acute myeloid leukemia (AML) is initiated and sustained by leukemia stem cells (LSCs) which arise from progenitor cells that do not usually self-renew but become aberrantly self-renewing. It is thought that LSCs gain aberrant self-renewal potential by co-opting molecular and cellular programs from hematopoietic stem cells (HSCs) (PMID: 16862118). HSCs have been shown to require tightly regulated protein synthesis rates, where increased or decreased protein synthesis impairs self-renewal (PMID: 24670665), but it is not known if LSCs share this dependence. We have shown that human LSCs reside in the population of AML cells with the highest levels of CD99 (PMID: 28123069). In RNA-sequencing studies, we found that LSCs with high levels of CD99 are depleted for ribosomal protein transcripts. We thus reasoned that similar to HSCs, LSCs may depend on tightly regulated protein synthesis to self-renew.
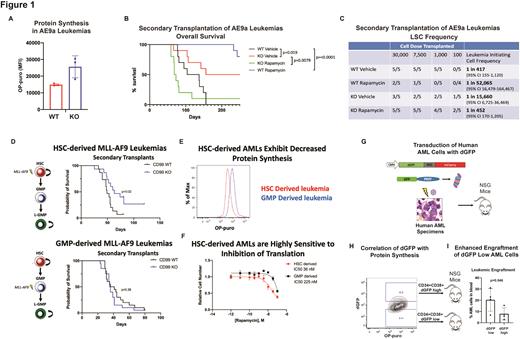
To test if CD99 promotes LSC function by constraining protein synthesis, we transduced c-Kit+ cells from B6-CD99 Gt(pU-21T)44lmeg (CD99 KO) or wild-type (WT) mice to express AML1-ETO9a (AE9a) and transplanted them into WT mice treated with rapamycin or vehicle. There was no difference in leukemogenesis in primary recipients, but CD99 KO-AE9a AMLs exhibited a 72% (p=0.048) increase in protein synthesis compared with WT-AE9a AMLs (Figure 1A), confirming that CD99 negatively regulates protein synthesis in AML. We next performed secondary transplants to assess LSC function, as measured by survival of secondary recipients in the absence of rapamycin treatment (Figure 1B). We furthermore performed these transplants at limiting dilution to quantify LSCs (Figure 1C). CD99 KO-AE9a vehicle treated AMLs demonstrated improved survival and a lower LSC frequency compared with WT-AE9a vehicle treated AMLs, consistent with a self-renewal defect with loss of CD99. Rapamycin treatment completely rescued this defect, leading to decreased survival and increased LSC frequency in CD99 KO-AE9a AMLs compared with vehicle. Conversely, rapamycin treatment depleted LSCs in WT-AE9a AMLs, increasing survival and decreasing LSC frequency compared with vehicle. Thus, similar to HSCs, LSCs are adversely affected by both increases or decreases in protein synthesis.
MLL-AF9-induced mouse AMLs initiated in HSCs as compared with granulocyte macrophage progenitors (GMPs) exhibit increased epigenetic imprinting of HSC features resulting in disease features reminiscent of high-risk AML (PMID: 23235717). To test if HSC-derived leukemias exhibit increased dependence on regulated protein synthesis, we transduced HSCs or GMPs from CD99 KO or WT mice to express MLL-AF9 and transplanted them into WT recipients, followed by secondary transplants to assess LSC function. Loss of CD99 led to increased survival indicative of decreased LSC function in HSC-derived but not GMP-derived leukemias (Figure 1D). This suggests that HSC-derived leukemias co-opt from HSCs a more pronounced dependence on tightly regulated protein synthesis. Accordingly, WT HSC-derived leukemias exhibited decreased protein synthesis as compared with their WT GMP-derived counterparts (Figure 1E), as well as increased sensitivity to rapamycin (Figure 1F).
To directly study protein synthesis in human LSCs, we transduced primary AML specimens (n=4) to express a destabilized form of GFP (dGFP) from a constitutive promoter followed by xenotransplantation (Figure 1G), allowing us to measure dGFP by flow cytometry as a surrogate for protein synthesis rates in vivo. We validated this assay by measuring protein synthesis using orthogonal O-propargyl-puromycin incorporation assays (Figure 1H). Human AML cells with low levels of dGFP demonstrated increased engraftment in secondary transplants (Figure 1I), demonstrating that human LSCs exhibit low protein synthesis rates.
In conclusion, our data demonstrate that LSCs co-opt from HSCs a dependence on tightly regulated protein synthesis. This is the first description of a cellular feature co-opted from HSCs that also represents a therapeutic vulnerability. Furthermore, the types of AML that exhibit the most robust re-activation of HSC programs and increased dependence on regulated protein synthesis are also likely to represent high-risk AMLs most resistant to standard therapies. Our data suggest that such therapy resistant AMLs may be highly sensitive to strategies disrupting protein synthesis to deplete LSCs.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal